|
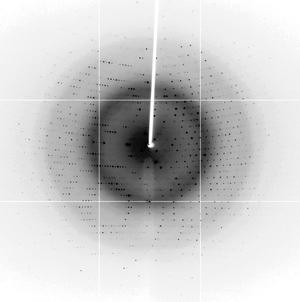
v-FLIP MC159 X-ray diffraction from data set at X29 beam line at NSLS-I |
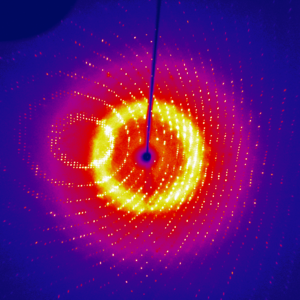
Diffraction image for tetragonal lysozyme using the RAXIS-IV++ detector and RuH3R home source |
For an example of what data collection on a well-diffracting crystal looks like, here's a video of a 90° data collection (100 sec/0.5°) crammed into 7 seconds from our older in-house source. Proteinase K is a strong diffractor and this data extends well past the edge of the detector at 1.6 Å resolution. You could collect that sort of data in 15 minutes on our current system.
For straightforward cases (reasonable diffractors, available homologous structures) we can determine structures using data collected in-house. For structures with enough sulfurs (Met, Cys) and strongly-diffracting crystals we can now attempt S-SAD experimental phasing in-house, or we can use other anomalous scatterers (Iodide, Ho3+ substituting for Ca2+, etc). For other cases (novel structures and/or weak diffractors) we must use the ultra-bright tunable X-ray sources at synchrotrons. In the last couple of years we've used the Cornell High Energy Synchrotron Source (CHESS) beam lines A1 and F1, and the AMX beamline of NSLS-II at Brookhaven National Lab.
For the in-house source we support the HKL3000 package for data collection and processing.
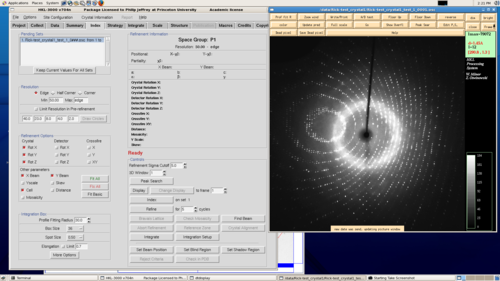
HKL3000. Click image for larger version
We can also collect small-molecule data sets to a maximum resolution of 0.83 Å on the new system - complete structures are usually possible in a couple of hours and initial structures possible within 10 minutes using the CrysAlisPro software.